Copyright Newsweek
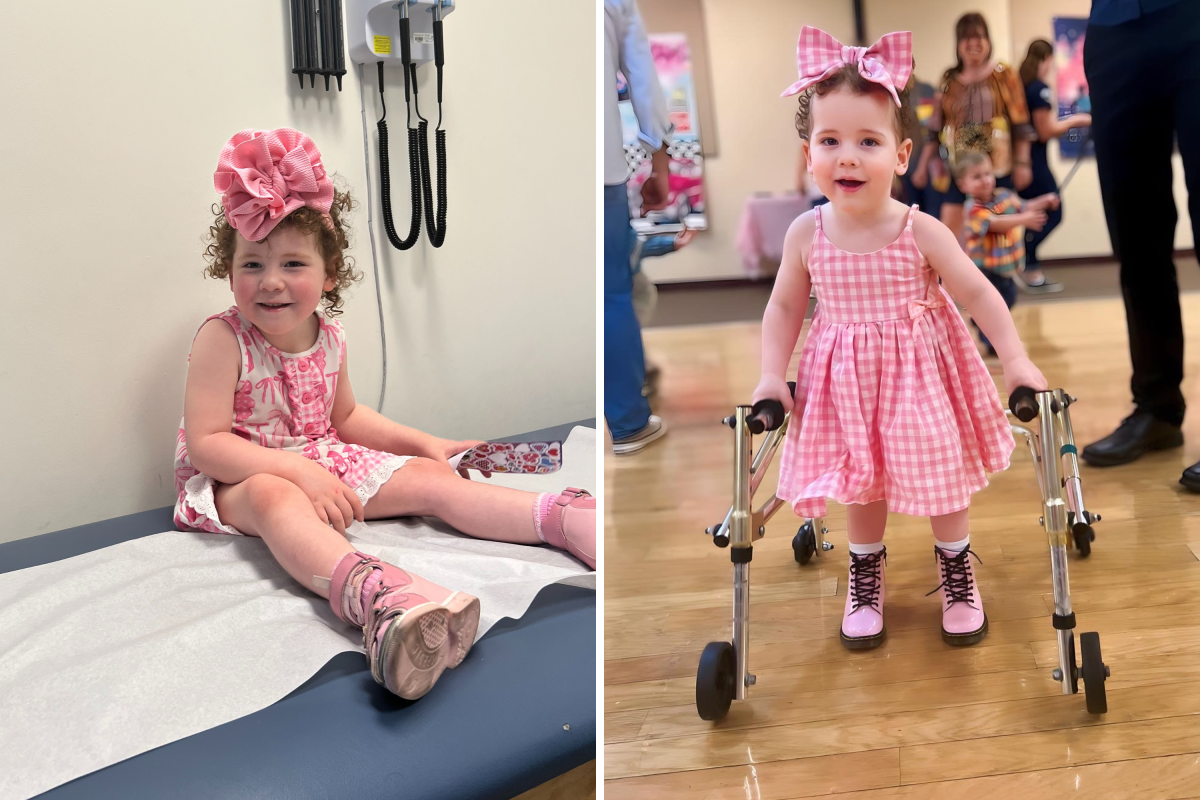
When four-year-old Harlow Prado woke up from anesthesia after her first dose of life-saving gene therapy, the first thing she asked was, “Mama, can I walk yet?” Harlow has a rare brain disorder called TUBB4A-related leukodystrophy, which affects her motor development. For her entire life, she has relied on a walker and attended eight therapy sessions a week. But on October 15, she became the first girl in the world to receive a personalized treatment for the disorder—one that could help her achieve her dream of walking. Speaking to Newsweek from her daughter’s bedside in the hospital, Daphne Graskewicz-Prado, from Southern Illinois, said: “I wasn’t going to accept the terminal diagnosis.” What Is TUBB4A-Related Leukodystrophy? TUBB4A leukodystrophy is an extremely rare and serious brain disorder caused by a mutation in the TUBB4A gene. According to Alex, The Leukodystrophy Charity, this gene helps produce a protein that maintains myelin, the protective coating found around nerve cells. Myelin acts like insulation on electrical wires, ensuring that signals travel quickly and accurately through the brain and body. When the gene is faulty, the resulting protein disrupts this protective coating. Without proper myelin, nerve signals slow down or get lost, damaging the brain’s white matter. This can lead to muscle weakness, movement difficulties, trouble eating and sometimes paralysis. Because the condition is so rare and its symptoms vary, it’s often misdiagnosed, requiring advanced genetic testing for confirmation. There is currently no cure for TUBB4A-related leukodystrophies. While some children with the most severe forms of the disease died in their late teens or early twenties, life expectancies can vary widely between different subtypes of the disorder. The Diagnosis Graskewicz-Prado first grew concerned when Harlow was 15 months old. “I knew something was wrong,” she said. “Harlow could pull herself up on furniture and cruise along objects, but she couldn’t stand or take those first steps on her own.” By 21 months, Harlow still was not walking. Doctors initially suspected cerebral palsy, but an MRI scan showed reduced myelin in key areas of her brain. These white matter changes, especially in regions that control balance and movement, pointed to something more severe. A seven-month wait for a neurology appointment felt impossible, so Graskewicz-Prado found a pediatric neurologist three hours away. Genetic testing later confirmed the diagnosis in July 2023. “We were told to get therapy while we have her,” she recalled. “But at the end of the conversation, the doctor mentioned a nonprofit that helps find treatments—and that was my only hope.” Without intervention, she explained, the progression of the condition would essentially leave Harlow: "locked in her body until she passed away.” Harlow Becomes First Girl to Receive This Groundbreaking Treatment Two months after the diagnosis, n-Lorem, a nonprofit that develops personalized therapies for ultra-rare genetic diseases, agreed to help. Based in Carlsbad, California, the organization funds free, lifelong experimental antisense oligonucleotide (ASO) treatments for patients with unique mutations affecting fewer than 30 people worldwide. ASOs are short, synthetic DNA strands designed to target specific gene mutations. They can precisely bind to RNA to block or correct faulty genetic messages—an especially valuable approach for “nano-rare” diseases where traditional drugs are not feasible. Harlow’s family still needed to raise $100,000 to cover administrative, research and staffing costs. In August 2024, the military family moved to Temecula, California and began crowdfunding. Within four days, they surpassed their goal. On October 15, Harlow received her first treatment. “When I got her diagnosis, I had this audacious thought—and through grit, love, support and luck, we did it,” said Graskewicz-Prado. “I know what our world would have looked like without this intervention. "I can’t wait to see her take the world by storm now that she’s had this medicine." Less than one week later, Graskewicz-Prado told Newsweek that therapists have noticed very subtle changes such as Harlow's hand tremors have reduced and she is not dragging her feet as much. Amy Williford, Ph.D., the Executive Director Communications and Donor Relations for n-Lorem Foundation, told Newsweek: “Harlow is the second person in the world to receive this medication and the first female.” As a non-profit organization, n-Lorem is committed to promoting health equity by ensuring that patients are not required to pay for their medicines. Instead, the organization provides all research, development and treatment efforts free of charge and guarantees lifelong access to the necessary medication at no cost to the patient. “Harlow represents the reason that n-Lorem was formed in 2020," Williford said. “We are not focused on a particular disease or gene, but rather on a patient population that has a single gene mutation that makes them the rarest of the rare, these patients are severely affected and in need of a medicine, there is no medicine available to treat their condition, they are truly alone in their disease. “We feel that they should not be left behind.” A Fighter With a Big Spirit Growing up surrounded by medical professionals, Harlow has developed a wisdom beyond her years. “She’s an incredible little person with a big spirit,” said her mother. “There’s no doubt she’s destined to do great things.” “My job as a mom is to give her the medicine, treatment, and therapy she needs so she can live the life she wants. The only thing holding her back is this condition.” Graskewicz-Prado has since co-founded the Kinslow TUBB4A Foundation, a nonprofit advocating for equitable treatment access for all children with TUBB4A mutations. “My dream is to make this treatment available to every mom,” she told Newsweek. “They love their kids as much as I love mine. We’re blessed to have this opportunity—I want other families around the world to have it too.”



