Copyright Reuters
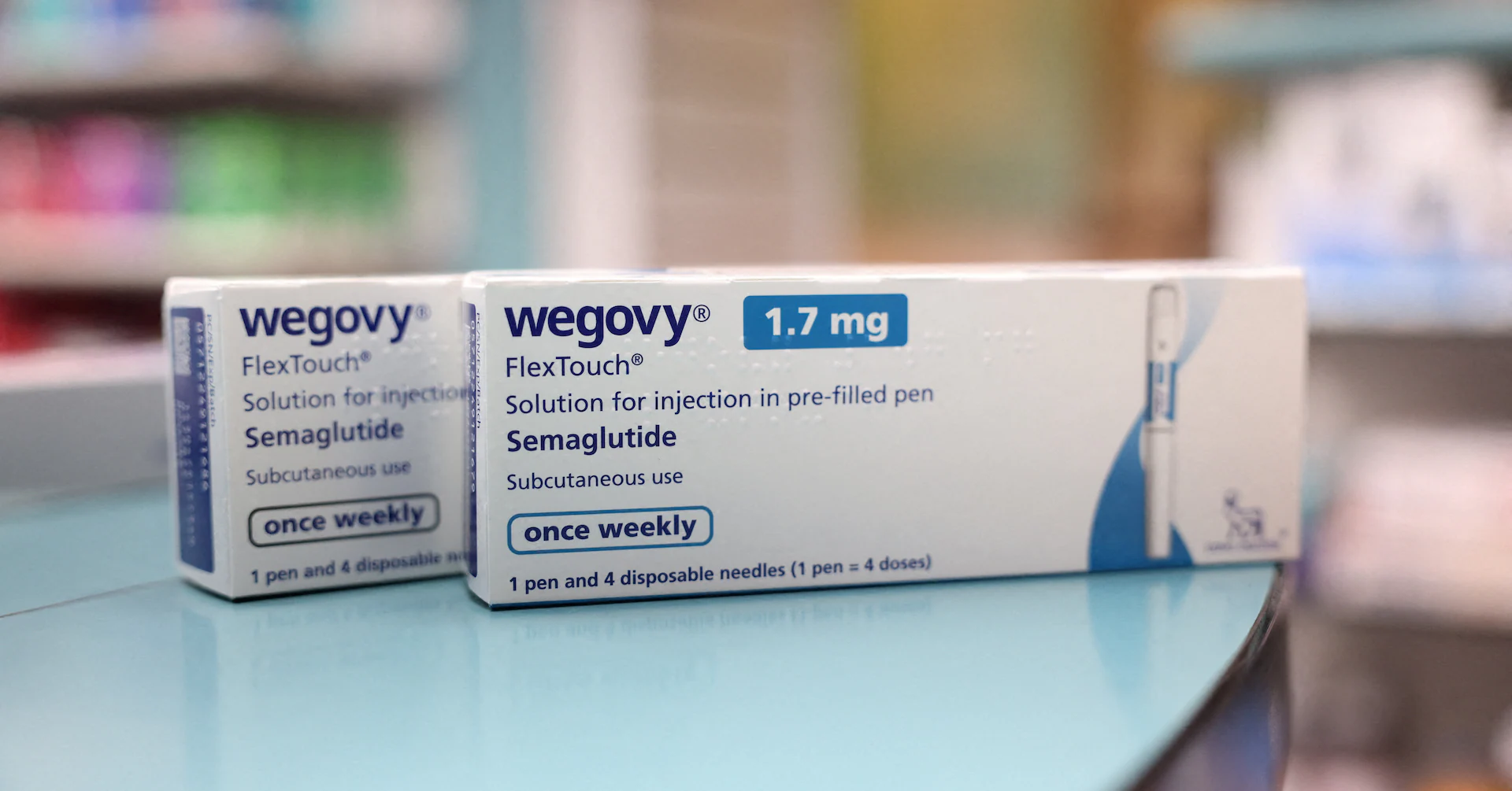
The collaboration is a potential boost for WeightWatchers as it emerges from bankruptcy and highlights its commitment to sell branded obesity drugs, unlike rivals that push cheaper copies in a highly competitive market. Sign up here. "We have been working with Novo Nordisk ahead of time to support the launch of oral weight-loss medication," WeightWatchers CEO Tara Comonte said in an interview. "A lot of people don't want an injection. And the convenience of a pill is going to be huge," she said. WeightWatchers could keep more customers on its platform than telehealth rivals, as doctors expect U.S. patients to switch to the branded medicines once prices come down under a deal announced last week by the White House. Lilly and Novo agreed to slash prices of their popular GLP-1 injectable drugs for U.S. government health programs and cash-paying customers. Starter doses of the weight-loss pills, if approved, will cost $149 a month for Medicare and Medicaid enrollees and for cash payers through the U.S. administration's new TrumpRx site. "Anything that brings pricing down for these medications is good for WeightWatchers," Comonte said. WeightWatchers is also trying to gain a stronger foothold in women's health, including through tailored programs that offer GLP-1 medicines and hormone replacement therapy. Outside of the U.S., Germany and the UK are major markets for WeightWatchers, which had 4 million subscribers worldwide at the end of 2023. Reporting by Bhanvi Satija in London; Editing by Bill Berkrot Our Standards: The Thomson Reuters Trust Principles., opens new tab Bhanvi is a London-based reporter covering European pharmaceutical companies and the healthcare industry. She previously covered U.S. health and pharma firms, with a focus on the new weight loss drugs that are transforming the obesity treatment space. Her coverage includes a trend piece on the underuse of their weight-loss drugs among men, increased interest in therapies being developed for preservation of lean mass, and a scoop on gene therapy maker Sarepta defying an FDA order to stop shipping its muscular dystrophy treatment.