Copyright Los Angeles Times
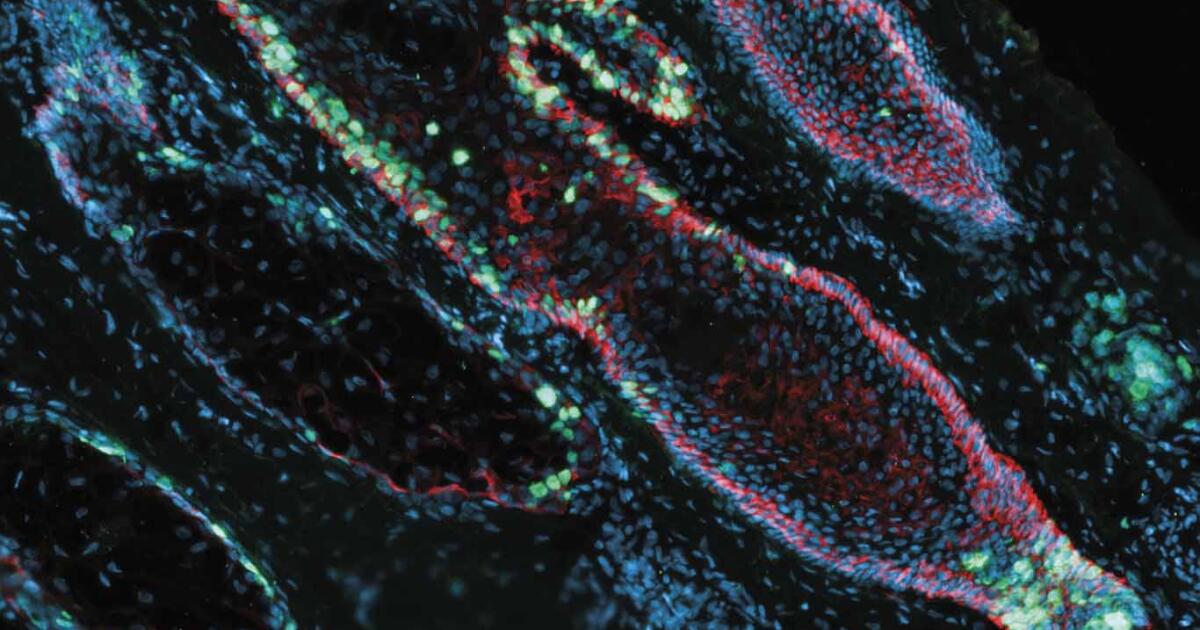
Pelage Pharmaceuticals, a clinical-stage regenerative medicine biotech firm based in Los Angeles, closed a $120 million Series B round co-led by ARCH Venture Partners and Google Ventures, with participation from existing investors, including Main Street Advisors, Visionary Ventures and YK Bioventures. The financing will support the continued advancement of Pelage’s lead program, PP405, a topical small molecule designed to reactivate dormant hair follicle stem cells, offering a potential first-in-class approach for both men and women experiencing hair loss. “At Pelage, our goal is to deliver a clinically tested treatment to combat hair loss, grounded in innovative science. Despite the growing need for treatments for hair loss, which affects more than 80% of men and 40% of women throughout their lifetimes, innovation has been lacking, and a new FDA-approved option has not become available for decades,” said Daniel Gil, chief executive of Pelage Pharmaceuticals, in a statement. “Our approach is based on the groundbreaking discovery that hair follicle stem cells have a unique metabolic switch. Supported by over 10 years of bench research on the mechanism and associated biological pathways, we have designed a first-in-class investigational treatment that reactivates these hair follicle stem cells to regrow hair,” added Gill. This new funding will support the company’s Phase 3 trials for lead program PP405 in 2026. In conjunction with the financing, Cathy Friedman, who led the company’s Series A financing, has been appointed chair of the board. Additionally, Richard Heyman, venture partner at ARCH, will join Pelage’s board of directors, alongside Daniel Gil and William Lowry, co-founder and president of Pelage. “Pelage is a pioneer in the regenerative medicine space, with a scientifically rigorous approach to address hair loss that sets them apart from currently available treatments. This could fulfill an urgent need, as we see a growing portion of the population experiencing hair loss, including women, who only have one FDA-approved option,” said Cathy Friedman, executive venture partner at Google Ventures, in a statement.



