Copyright lestimes
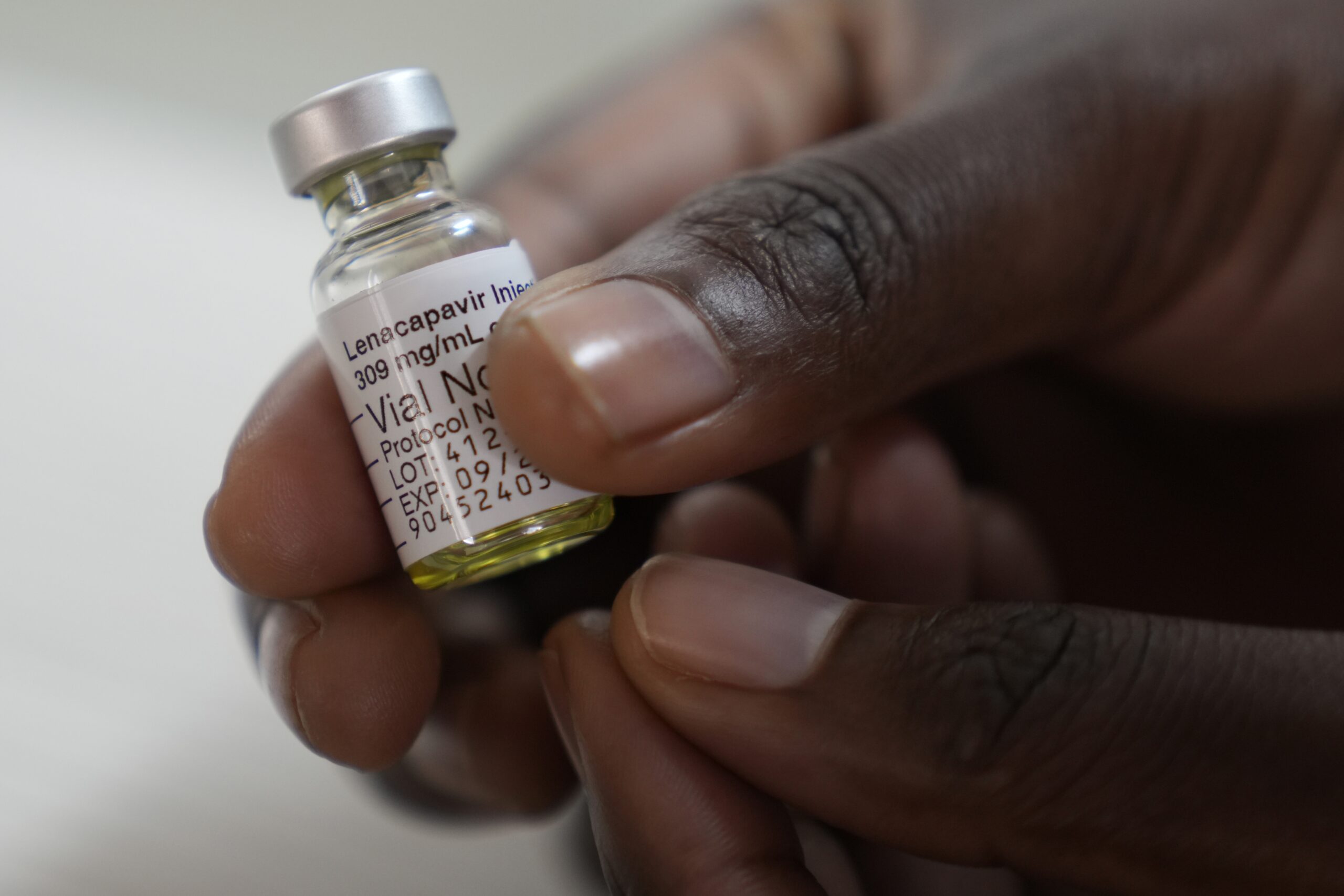
Mathatisi Sebusi THE Ministry of Health will next week sign an agreement with the United States government to pilot a newly developed HIV prevention drug, lenacapavir, which requires only two injections per year. Following the signing, Lesotho will begin rolling out lenacapavir — a long-acting injectable developed by US-based Gilead Sciences — as part of a groundbreaking HIV prevention programme under the President’s Emergency Plan for AIDS Relief (PEPFAR). The initiative will be jointly funded by PEPFAR and the Global Fund. Minister of Health, Selibe Mochoboroane, said this in an exclusive interview with the Lesotho Times this week. Mr Mochoboroane said the agreement marked renewed US commitment to supporting Lesotho’s HIV response, following a period of reduced funding. “Recently, I visited the United States where I met with government representatives. Among the key issues discussed was Lesotho piloting the administration of lenacapavir,” he said. “We agreed that the US government will introduce this kind of vaccine. The US also proposes the ‘America First Global Health Strategy,’ which is built on three pillars — making America safe, great, and prosperous.” He explained that the “make America safe” pillar includes helping countries like Lesotho to prevent infectious diseases to reduce global health risks. However, continued US assistance is conditional on countries’ performance in previous aid programmes such as PEPFAR. Mr Mochoboroane said Lesotho’s outstanding progress in the HIV response has strengthened its eligibility for inclusion in the lenacapavir pilot. “Globally, the HIV target is 95-95-95 by 2030 — meaning 95% of people living with HIV should know their status, 95% of those who know their status should be on treatment, and 95% of those on treatment should have suppressed viral loads,” he explained. “We have surpassed these global targets. Lesotho is at 97-97-99.” He added that by 2030, countries are also expected to reduce new HIV infections by 90%. Lesotho currently stands at 87%, showing significant progress toward that goal. Mr Mochoboroane said Lesotho’s strong tuberculosis (TB) response was another factor in its selection for the lenacapavir pilot. “Global targets require countries to reduce the TB burden by 90% by 2030. Lesotho is on track, increasing TB notifications and ensuring patients receive treatment. This puts us in a strong position — among the 16 countries selected by the US government to continue working with,” he said. The minister added that the US will also assist Lesotho in strengthening human resources for health, particularly by increasing the number of frontline healthcare workers. “I have received a letter from the US government informing me that their delegation will arrive in Lesotho in the first week of November to negotiate and sign the new deal,” he said. In August 2025, the US Department of State announced plans to make lenacapavir available in countries with high HIV burdens, including Lesotho. According to the Department’s statement, lenacapavir is a highly effective HIV prevention medication that requires administration only twice a year. The rollout will prioritise pregnant and breastfeeding women who face a higher risk of HIV transmission. A large-scale clinical trial demonstrated remarkable results, with over 99% of participants remaining HIV-negative while on lenacapavir. The initiative aims to reach up to two million people by 2028, with allocation depending on each country’s HIV burden and capacity to distribute and administer the drug effectively. “In the coming months, PEPFAR will collaborate with partner countries to develop detailed rollout strategies, prioritising high-risk groups — especially mothers and infants,” the statement said. US Senior Official for Foreign Assistance, Humanitarian Affairs and Religious Freedom, Jeremy Lewis, said lenacapavir’s twice-yearly dosage offers major advantages. “The dosage schedule increases convenience and adherence, helping reduce transmission rates and lower treatment costs,” he said. “The US will continue partnering with countries with large HIV epidemics to co-develop targeted distribution plans, with a strong emphasis on preventing mother-to-child transmission.” Global Fund Executive Director, Peter Sands, emphasised the importance of fast-tracking access to medical innovations like lenacapavir. “We must ensure breakthrough tools are introduced quickly, affordably, and where they will have the greatest impact,” he said. “By focusing on regions where lenacapavir can make the biggest difference — and working closely with the US and Gilead — we can help countries integrate this innovation into their HIV prevention programmes. This will reduce new infections and accelerate progress toward self-reliance and epidemic control.”



