Copyright newskarnataka
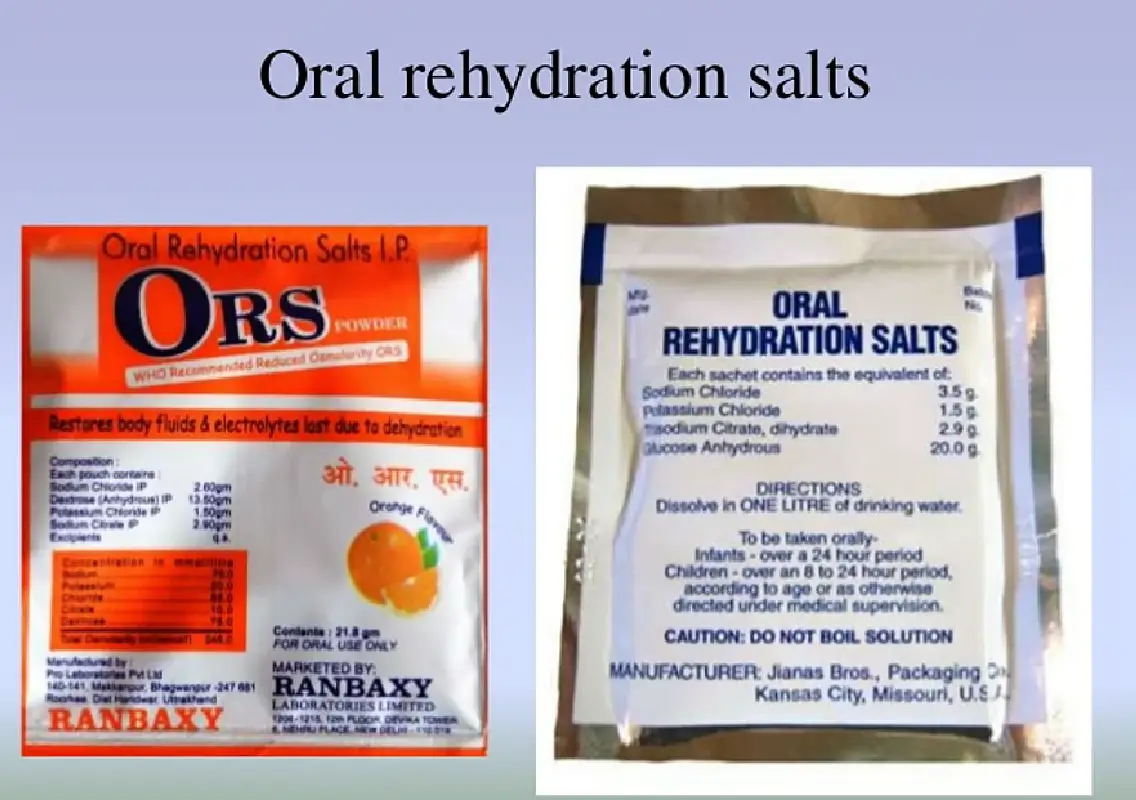
The Delhi High Court on Tuesday (October 28) issued a crucial clarification on its earlier order concerning beverages falsely marketed under ‘ORS’ (Oral Rehydration Solution) labels, following widespread social media misinterpretation of its October 17 ruling. Justice Sachin Datta emphasised that the previous order was not a blanket permission for manufacturers to continue selling ORS-labelled drinks, and that such an understanding was “completely misplaced.” “It wasn’t to allow manufacturers to continue production. The idea was for FSSAI to decide representations within two to three days,” Justice Datta stated, expressing sharp displeasure at the regulator’s delay in acting on the issue. The clarification came during a hearing on a petition by Dr Reddy’s Laboratories, which has challenged the Food Safety and Standards Authority of India’s (FSSAI) October 14 and 15 directives restricting the use of “ORS” in product branding. Background: The ORS Trademark Ban The FSSAI withdrew earlier approvals that had allowed the use of the term “ORS” — even with prefixes or suffixes — in trademarks for hydration and electrolyte drinks, citing public health risks. The crackdown followed an eight-year campaign by Hyderabad-based paediatrician Dr Sivaranjani Santosh, who documented how “fake ORS” drinks misled parents and endangered children suffering from diarrhoea and dehydration. The authority stated that only formulations strictly matching WHO standards qualify as genuine oral rehydration solutions. Court’s Stern Message to FSSAI Justice Datta warned the regulator against further delay: “If you [FSSAI] are unable to decide by Friday, move an application. I may pass orders restraining manufacturers from producing these beverages.” The court also took note of social media posts misrepresenting the October 17 order as permission for companies like JNTL Consumer Health India (maker of ORSL) to resume production, and categorically rejected such interpretations. New Petitions and Industry Response Dr Reddy’s Laboratories, whose hydration drink Rebalanz VITORS is under scrutiny, argued that it includes proper disclaimers and follows WHO guidelines. The company contended that FSSAI’s order violated due process by being issued without consultation or hearing. Meanwhile, JNTL Consumer Health, in its defence, said the sudden withdrawal of approval affects ₹155–180 crore worth of stock and damages brand equity built over two decades. Health Experts Sound Alarm Medical experts warn that most commercial beverages labelled as “ORS” contain 8–10 times more sugar and lack essential electrolytes such as sodium and potassium. “Even if the label says ‘not ORS,’ the branding confuses parents,” said Dr Santosh, noting that diarrhoea still accounts for 13% of deaths in children under five in India, as per NFHS-5 data. Doctors urge parents to use WHO-approved ORS sachets only, as sugary drinks can worsen dehydration by disrupting the osmotic balance. The High Court will next hear the matter on October 31, 2025, reviewing FSSAI’s compliance and the merits of Dr Reddy’s petition. The outcome is expected to shape the future of trademark use, food labelling, and health safety regulations in India’s ₹10,000-crore beverage sector.



