Confusion over vaccine recommendations in the U.S. has hit a boiling point as the responsibility for handling them has spilled onto individual states. Regulators at the Centers for Disease Control and Prevention and the Food and Drug Administration have long provided trusted national guidance on who should get certain vaccines and when. But now reports of non-evidence-based policy changes at the federal level, along with fears of reduced access, have led a handful of states to start implementing their own new vaccine policies and programs.
At center stage: COVID vaccines.
Since taking office, Secretary of Health and Human Services Robert F. Kennedy, Jr.—a longtime supporter of the antivaccine movement—has made a series of alarming moves that have thrown the federal vaccine recommendation process into turmoil. Kennedy removed the COVID shot from the CDC’s vaccine schedule for pregnant people and healthy children without input from the Advisory Committee on Immunization Practices (ACIP), an independent panel of experts that has advised the CDC since 1964. Then, last month, he announced that the FDA had approved the 2025–2026 COVID vaccine only for people aged 65 and older and those with certain underlying health conditions. This means that people who do not fall into these eligible groups may need to obtain a prescription or pay a hefty out-of-pocket bill. The situation could change again, however, because ACIP will vote on recommendations for vaccines for COVID and other illnesses this week.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Amid the chaos, some states are forging ahead individually or in coalitions. Several have authorized pharmacists to administer the COVID vaccine and/or have required insurers to cover the cost.
“States shouldn’t have to invent their own systems,” says Northe Saunders, president of American Families for Vaccines, a national nonprofit organization that campaigns for science-based public health laws. “For decades, ACIP has been the rigorous, independent body we could all rely on to make evidence-based vaccine recommendations. That process wasn’t broken—RFK, Jr., broke it by injecting politics where only science belongs.”
In June Kennedy fired all 17 members of ACIP and, days later, appointed eight new, handpicked ones, some of whom have expressed antivaccination views. The Department of Health and Human Services announced five more committee members on Monday, ahead of this week’s meeting. ACIP’s recommendations must be formally adopted by the CDC’s director; currently, HHS Deputy Secretary Jim O’Neill is serving as acting CDC head after HHS and White House officials fired the agency’s then director Susan Monarez, reportedly for refusing to “rubber-stamp unscientific” health and vaccine policies, in the words of her attorneys. Monarez appeared before a Senate hearing on Wednesday about her dismissal.
In an e-mail responding to questions over Kennedy’s actions with ACIP from Scientific American, HHS communications director Andrew Nixon wrote, “Under the old ACIP, outside pressure to align with vaccine orthodoxy limited asking the hard questions.” Kennedy has claimed the former ACIP members were biased and had conflicts of interest. Historically members have abstained from meeting deliberations and voting that involves any product over which they have disclosed a conflict of interest.
Federal vaccine guidance for the U.S. public will continue to be a moving target in the coming days, but experts offer some clarity on how new state orders may affect your access to COVID shots.
Fallout from the FDA Decision
The FDA’s decision to limit COVID vaccine access trickles down in two major ways: First, in seven states (as of publication) that require ACIP approval for pharmacists to give vaccines, people need a clinician’s prescription for a COVID vaccination at a pharmacy. This will likely add a significant barrier to access, given that nearly 90 percent of COVID vaccines were given at pharmacies during the 2024–2025 season. Such changes highlight a weakness in the historical strategy around pharmacy vaccine policies, says Brooklyn Morgan, a pharmacist and director of state policy at the National Alliance of State Pharmacy Associations (NASPA). “For years, many states linked pharmacist vaccination authority to ACIP recommendations, which was considered one of the best ways to ensure scope of practice kept pace with scientific innovation. However, the recent instability has shown that this approach leaves pharmacists and patients vulnerable to abrupt changes,” Morgan says.
Second, nearly all health insurance plans (including those offered or supported by the federal government) traditionally base vaccine coverage decisions on ACIP recommendations. America’s Health Insurance Plans (AHIP), the national trade association representing the health insurance industry, announced on Tuesday that private health insurance plans will continue to fully cover new COVID vaccines. People with private insurance who fall into eligible groups for these vaccines—or who live in a state with that issued orders to expand eligibility requirements—will not have to pay for such vaccinations through the end of 2026. (A COVID shot for an adult can cost $150 to $200 out of pocket.)
Some states (listed below) are addressing this issue themselves by requiring insurers to pay for the COVID vaccines. And some insurers may opt to pay for them regardless of ACIP’s recommendations; a vaccination costs far less than a complicated COVID illness. Before making an appointment, check with your insurance company to avoid unexpected costs.
States Join Forces
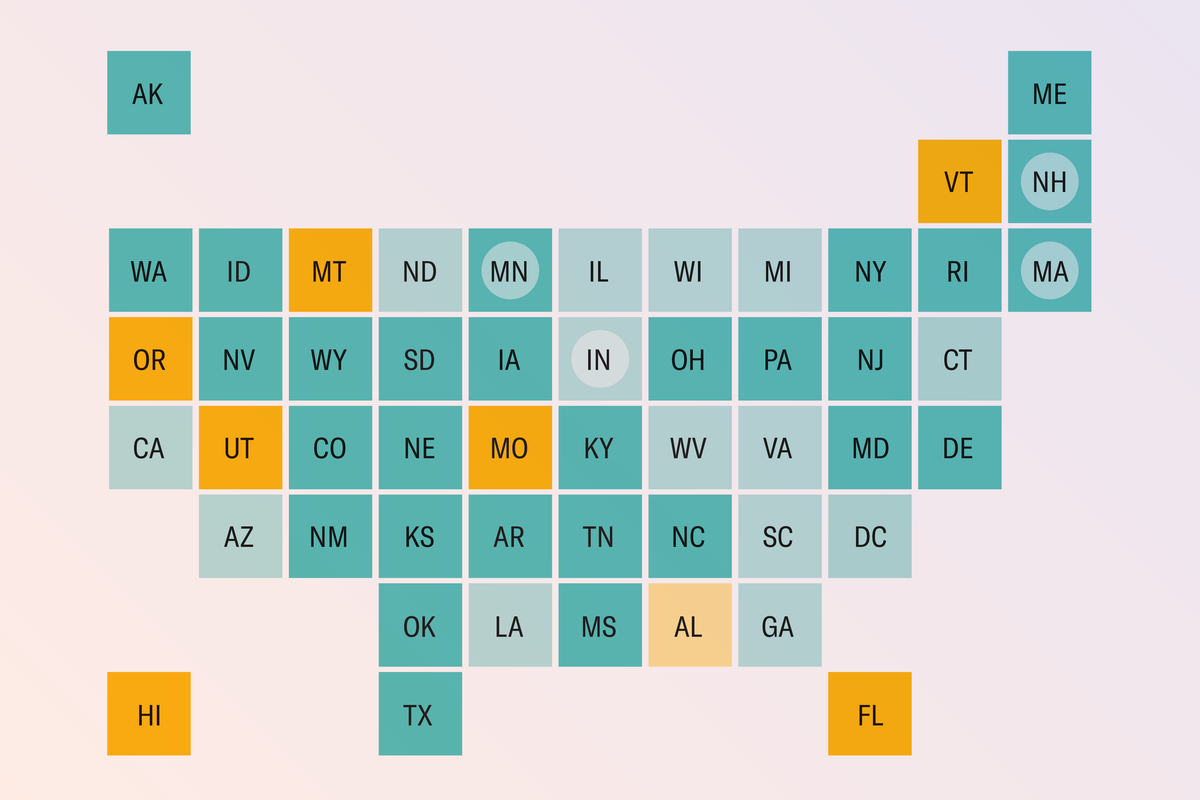
California, Oregon, Washington State and Hawaii have joined the West Coast Health Alliance, which will develop its own vaccine guidelines for COVID and other respiratory illnesses, independent of the CDC or the FDA. (In Oregon, however, the COVID vaccines remain unavailable at pharmacies without a prescription—and many hospitals and doctors are reluctant to provide one, according to local reports.)
Erica Pan, director and state public health officer at the California Department of Public Health, says the West Coast Health Alliance’s recommendations will be informed by guidance from several professional medical organizations, including the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists and the American Academy of Family Physicians. Experts in communicable diseases and immunizations will meet to review the organizations’ guidance, along with other scientific research.
“Our goal is to provide clear recommendations based in science while prioritizing the protection of our communities,” says Pan, who is involved in the West Coast Health Alliance.
A similar coalition is in the works on the opposite coast. Public health officials from Maine, Vermont, Massachusetts, Rhode Island, Connecticut, New York State, New Jersey, Pennsylvania and Delaware have met to discuss creating their own vaccine recommendations as a group.
Meanwhile participating states’ governors have issued executive orders to preserve COVID vaccine access—though directives currently vary by state. For instance, New York State’s order allows pharmacists to administer COVID vaccines at no cost; New Jersey’s cannot guarantee coverage by all insurance plans. Those seeking shots should check individual state guidance on eligibility, access and costs.
Individual State Variability
To date, Arizona, Colorado, Illinois, Maryland, New Mexico and Virginia have individually taken action to expand COVID vaccine access by allowing pharmacists to administer the vaccine under certain circumstances. Those circumstances vary by state and, in some cases, by pharmacy—so check with your state health department.
Other states haven’t taken steps to expand vaccine access or have announced piecemeal exceptions. New Hampshire is recommending pregnant people get the COVID vaccine, in contrast to the FDA recommendations. North Carolina and Virginia are allowing pharmacists to give shots without a prescription to those who meet the FDA requirements.
On the far end of the spectrum is Florida. Recently, Florida Surgeon General Joseph Ladapo said he has a goal to end the state’s availability of mRNA COVID vaccines (both Pfizer’s and Moderna’s COVID vaccines are mRNA-based). This move, on top of Ladapo’s recent decision to end all vaccine mandates in the state, may make it even more difficult for Florida residents to get a COVID vaccination.
Currently, no federal statute prevents people from traveling to another state to receive a COVID vaccine. Individual states have their own policies and restrictions, however—and where you get the shot may affect insurance coverage. If you plan to travel across state lines, check with the pharmacy in that state as well as your insurance company.
A Patchwork of Vaccination Coverage
This week the newly reconstituted ACIP is expected to make recommendations on the measles, mumps, rubella and varicella (MMRV), hepatitis B and COVID vaccines. News reports suggest that the panel plans to review an unverified report linking the deaths of 25 children to the COVID vaccines and to examine the vaccines’ safety during pregnancy. “FDA and CDC staff routinely analyze [Vaccine Adverse Event Reporting System] and other safety monitoring data, and those reviews are being shared publicly through the established ACIP process. Until that is shared publicly, any of this should be considered pure speculation,” Nixon wrote in an e-mail in response to questions from Scientific American.
The outcomes of the ACIP votes could pit Kennedy’s HHS against state governors regarding areas far beyond COVID.
Jessica Malaty Rivera, an infectious disease epidemiologist, says she’s anxious about the upcoming ACIP meeting. “I hate to speculate, but I fully expect ACIP and [acting CDC director] O’Neill to endorse the decisions RFK, Jr., and the FDA have already set in motion,” she says. “I also anticipate a terrible decision on the hepatitis B birth dose and possibly [on] MMRV.”
If state coalitions and individual states continue to make their own recommendations, independent of national health agency guidance, the resulting vaccine coverage mishmash could have profound consequences on U.S. public health. Unvaccinated or undervaccinated communities increase the risk of vaccine-preventable disease outbreaks of diseases other than COVID, such as measles, flu and other illnesses, Morgan explains. And policies are key to preventing these localized vulnerabilities.
“A patchwork of vaccination coverage risks undermining national public health goals,” Morgan says. “When recommendations and access vary widely by state, it can leave gaps in protection and create confusion among patients—which ultimately reduces overall vaccination rates.”



