By Paige Cockburn,Stephanie Dalzell
Copyright abc
A promising new Alzheimer’s drug has been green-lit by the national medicines regulator, after two previous failed attempts to register it for use in Australia.
Lecanemab, sold under the brand name Leqembi, has been shown to clear amyloid plaque in the brain, which experts believe plays a role in Alzheimer’s disease.
The drug is now registered for use by some patients with a diagnosis of mild cognitive impairment and mild dementia due to Alzheimer’s disease.
It had already been approved in around 50 countries, including the US, UK and China.
A phase-3 trial involving about 1,800 patients with early Alzheimer’s disease, published in the New England Journal of Medicine in 2022, showed that those who received the drug had reduced markers of amyloid plaque and less cognitive decline after 18 months than those who took a placebo.
Chris Rowe, director of the Australian Dementia Network at the University of Melbourne, has been running some of the Lecanemab trials in Australia and said the drug can reduce cognitive decline by around 30 per cent.
“What that means is that after the usual 18 months of treatment, you’ve deferred progression of the disease by about six months,” he said.
“But they’ve continued some studies for about four years and see that gain increases to about one year.”
In October last year, the Therapeutic Goods Administration (TGA) made an initial decision not to approve Lecanemab, arguing that safety and efficacy had only been established for some patients.
The TGA found safety had not been satisfactorily established for patients who carry one copy of the APOE4 gene — which increases the risk of developing Alzheimer’s disease — and neither safety nor efficacy had been established for those who carry two copies of the gene.
Professor Rowe said the side effects of Lecanemab can be three times as common for people with the APOE4 gene.
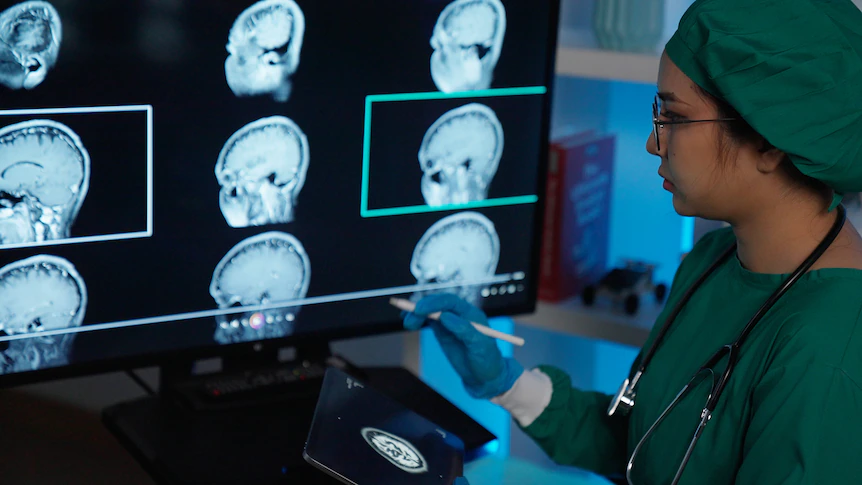
The most notable side effect of receiving Lecanemab is swelling or small bleeds in the brain, which can occur in around 10 per cent of people without the APOE4 gene.
However, most people do not notice any symptoms related to swelling or bleeding. Because of that, frequent MRI scans are conducted during treatment so doctors can monitor for any abnormalities.
Professor Rowe said there was less than a 1 per cent chance of developing a serious side effect from Lecanemab.
In a statement released today, the TGA said the Japanese drug manufacturer Eisai Aust was ultimately able to address the regulator’s safety concerns through “review and detailed discussions,” and the drug was registered only for non-carriers of the APOE4 gene.
Professor Rowe stressed that Lecanemab, which was given as a fortnightly intravenous infusion, was only for people in the early stages of the disease.
“The majority of people with dementia won’t qualify for this drug because they will be too advanced. What this does is put an increased emphasis on early diagnosis,” he said.
“So I strongly urge anybody with noticeable memory decline to see their doctor and get assessed early. Don’t put it off because this drug doesn’t work once you get past the mild stage of dementia.”
The other barrier is cost — a year’s worth of Lecanemab costs $39,974 in Australia, but Professor Rowe said that could quickly add up to $100,000, as patients must pay specialists to administer the infusions and have frequent MRI scans.
That means only a small pool of people will have the means to access the treatment unless it is listed on the Pharmaceutical Benefits Scheme (PBS), where it would be available at a heavily subsidised price.
Donanemab, another Alzheimer’s drug that clears amyloid, was approved by the TGA earlier this year but did not make it onto the PBS as its potential benefits were deemed too small to justify the burden on the health system.
Professor Rowe hoped things could be different for Lecanemab as patients were “desperate” to get access.
“One of the reasons the PBS knocked back Donanemab is because they claimed it only gave six weeks’ benefit … but with Lecanemab, all the tests consistently show a six-month benefit, so it may have a better shot of getting PBS approval.”



