Gunze Medical and HistoSonics Enter into Exclusive Distributor Agreement in Japan for Noninvasive Platform and Proprietary Sonic Beam Therapy Using Histotripsy Technology
By Business Wire
Copyright financialpost
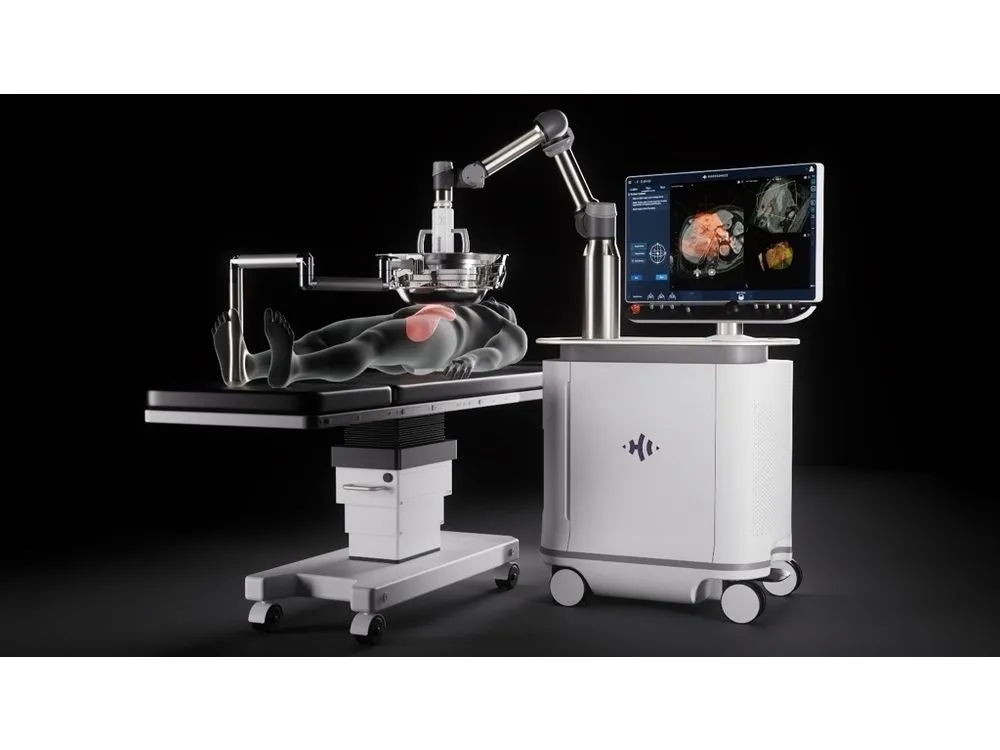
This medical device uses histotripsy technology, the world’s first noninvasive treatment method that utilizes microbubbles generated by ultrasound. Histotripsy is a novel technology that uses ultrasound energy to physically disrupt cancerous tissue without the need for surgical intervention. Focusing ultrasound at a single point creates a cluster of microbubbles called a “bubble cloud” within water-rich tissue. The rapid expansion and collapse of this bubble cloud precisely and selectively destroys and liquefies cancerous tissue. The destroyed cells are then excreted from the body as waste. A major advantage is the minimal damage to surrounding blood vessels and healthy tissue. Conventional treatments for liver cancer, such as surgery and radiofrequency ablation (RFA), have been the mainstream approaches. However, many patients have difficulty undergoing treatment due to factors such as impaired liver function, tumor location, complications, or advanced age. Histotripsy is gaining attention in Europe and the United States as a groundbreaking technology that offers a new treatment option for these patients. In Japan, histotripsy is currently approved to treat liver tumors, including hepatocellular carcinoma and metastatic liver cancer. However, it is expected to be used in the future to treat a wide range of diseases, including kidney and pancreatic cancers and benign prostatic hyperplasia. The Histotripsy platform received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) in 2021 and obtained formal market clearance in 2023. It has already been introduced at renowned university hospitals in the US and has accumulated over 2,000 treatment cases. In Europe, efforts are underway to obtain CE marking. In Asia, approval is planned for Japan, South Korea and Taiwan.